Amantadine PK & PD: Complete Guide to Pharmacokinetics and Pharmacodynamics
Oct, 18 2025
Amantadine Renal Dosing Calculator
Renal Function Assessment
Adjust amantadine dosing based on creatinine clearance (CrCl)
Dosing Recommendation
Based on renal function and article guidelines
Enter your values to see dose adjustment recommendations
Ever wondered how amantadine works inside your body and why it’s used for both viral infections and movement disorders? This article breaks down the drug’s journey from ingestion to action, covering every step of its pharmacokinetic (PK) profile and pharmacodynamic (PD) mechanisms. By the end, you’ll know exactly how the molecule is absorbed, distributed, metabolized, and eliminated, plus the neuro‑chemical tricks it pulls to treat Parkinson’s disease and combat influenza.
Key Takeaways
- Amantadine is rapidly and well absorbed after oral dosing, with an oral bioavailability of about 90%.
- It crosses the blood‑brain barrier efficiently, reaching therapeutic concentrations in the CNS within an hour.
- Renal excretion is the primary elimination route; dose adjustment is needed for impaired kidney function.
- Mechanistically, amantadine blocks NMDA receptors, enhances dopamine release, and interferes with influenza A viral M2 ion channels.
- Therapeutic drug monitoring (TDM) is seldom required but can guide dosing in renal failure or when drug interactions are suspected.
What is Amantadine?
Amantadine is a synthetic adamantane derivative that was first introduced in the 1960s as an antiviral agent against influenza A. Over the decades, clinicians discovered its dopaminergic activity, making it a mainstay for early‑stage Parkinson’s disease and drug‑induced extrapyramidal symptoms. Chemically, it is C10H17N, a small, lipophilic molecule that easily slips through cell membranes, including the blood‑brain barrier. Its dual antiviral and antiparkinsonian properties stem from distinct molecular targets, which we’ll explore in the sections that follow.
Pharmacokinetics Overview
Understanding the PK profile of amantadine is essential for dosing, especially in patients with renal impairment or those taking interacting medications. Below we walk through each phase of the drug’s ADME journey.
Absorption
Amantadine is administered orally, typically as a tablet or oral solution. After ingestion, peak plasma concentrations (Cmax) appear within 1-3 hours, reflecting rapid gastrointestinal absorption. Food can modestly delay Tmax but does not significantly affect the overall exposure (AUC). The drug’s high oral bioavailability-approximately 90%-means that the administered dose reaches the systemic circulation almost unchanged.
Distribution
Being lipophilic, amantadine distributes widely throughout body water compartments. The volume of distribution (Vd) ranges from 40 to 50 L/kg, indicating extensive tissue uptake. Crucially, the molecule penetrates the central nervous system (CNS) by traversing the blood‑brain barrier (BBB). Cerebrospinal fluid (CSF) concentrations reach roughly 70% of plasma levels, which underpins its efficacy in Parkinson’s disease.
Metabolism
Unlike many CNS agents, amantadine undergoes minimal hepatic metabolism. Less than 5% of the administered dose is transformed by cytochrome P450 enzymes, primarily CYP2D6, into an inactive metabolite. This low metabolic burden reduces the risk of enzyme‑mediated drug interactions, although caution is still advised when co‑administering potent CYP2D6 inhibitors.
Elimination
Renal excretion dominates amantadine clearance, accounting for about 80-90% of the dose. The drug is eliminated unchanged via glomerular filtration and tubular secretion. The mean elimination half‑life (t½) in healthy adults is 15-16 hours, which supports once‑ or twice‑daily dosing regimens. In patients with reduced creatinine clearance (<50 mL/min), the half‑life can extend beyond 30 hours, necessitating dose reductions to avoid accumulation and toxicity.
PK Summary Table
| Parameter | Value (Healthy Adults) | Influencing Factors |
|---|---|---|
| Oral Bioavailability | ~90% | Food modestly delays Tmax |
| Peak Plasma Time (Tmax) | 1-3 h | Formulation (tablet vs solution) |
| Volume of Distribution | 40-50 L/kg | Body water content, age |
| Half‑Life (t½) | 15-16 h | Renal function, age |
| Renal Clearance | ≈80% of dose | Creatinine clearance |
Pharmacodynamics Overview
Amantadine’s therapeutic actions arise from three main mechanisms: NMDA‑receptor antagonism, facilitation of dopamine release, and inhibition of the influenza A virus M2 ion channel. Each pathway contributes to its clinical uses.
NMDA‑Receptor Antagonism
By binding to the open channel of the N‑methyl‑D‑aspartate (NMDA) receptor, amantadine reduces excitatory calcium influx. This neuroprotective effect can dampen overstimulation that leads to dyskinesias, which partly explains its utility in managing levodopa‑induced motor complications.
Dopamine Release and Reuptake Inhibition
Amantadine promotes the release of dopamine from presynaptic terminals and weakly inhibits dopamine reuptake. The net increase in extracellular dopamine improves motor function in Parkinson’s disease, especially in early‑stage patients where dopaminergic neurons are only partially depleted.
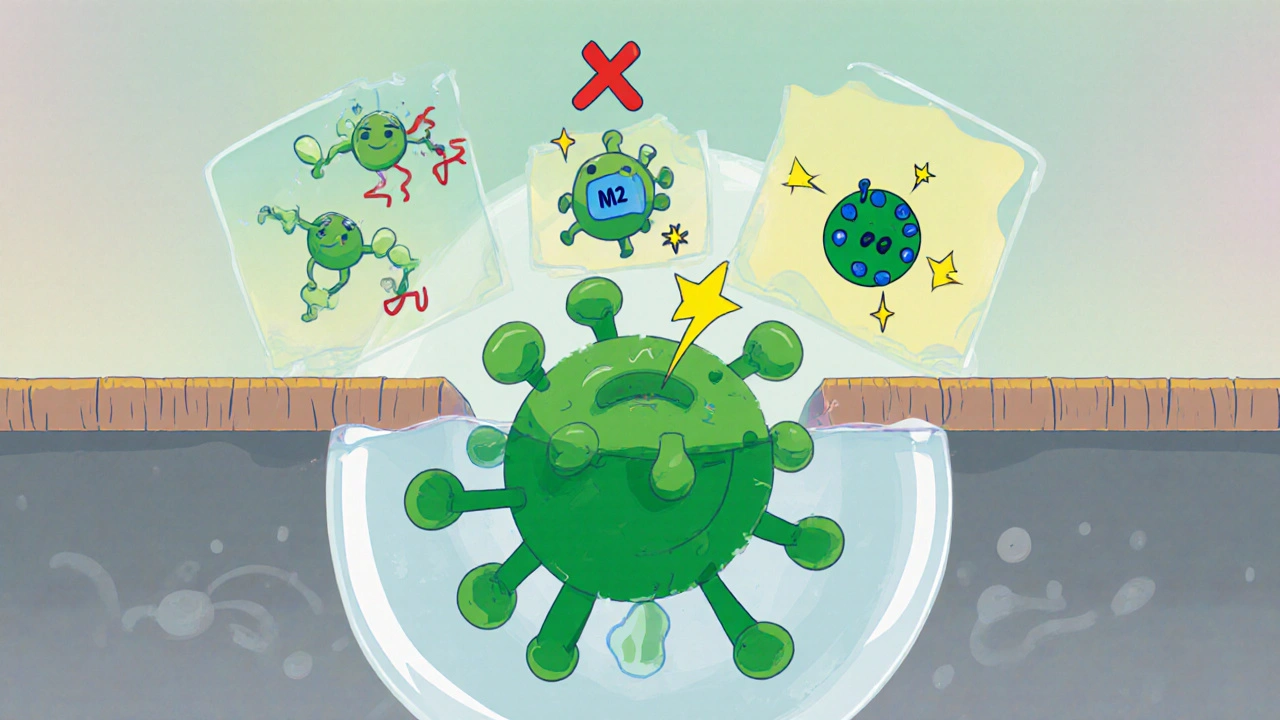
Antiviral Action Against Influenza A
The drug blocks the M2 proton channel of the influenza A virus, preventing acidification of the viral interior during endocytosis. This interruption halts viral uncoating and replication. Though resistance has limited its widespread use, amantadine remains a valuable option during specific outbreak strains lacking resistance.
Clinical Uses and Dosing
Two primary indications dominate contemporary prescribing:
- Parkinson’s disease: Initiated at 100 mg once daily, titrated up to 200 mg twice daily based on symptom control and tolerability.
- Influenza A prophylaxis or treatment: 200 mg once daily for prophylaxis; 200 mg twice daily for 5‑day treatment courses.
In pediatric patients, especially for viral prophylaxis, dosing is weight‑based (5 mg/kg/day). For elderly patients or those with renal impairment, a 50% dose reduction is commonly recommended.

Therapeutic Drug Monitoring & Safety
Routine TDM is not mandatory for amantadine, but plasma level checks become useful when renal function is compromised or when patients experience adverse effects despite standard dosing.
Adverse Effects
Common side effects include dry mouth, insomnia, and gastrointestinal upset. CNS‑related events-such as confusion, hallucinations, and peripheral edema-are more frequent in the elderly or those with renal failure. Severe toxicity can manifest as arrhythmias or seizures, underscoring the need for dose adjustment in high‑risk groups.
Drug Interactions
Because amantadine is minimally metabolized, it has few metabolic interactions. However, drugs that affect renal tubular secretion (e.g., probenecid) can raise amantadine levels. Concurrent use with other dopaminergic agents may amplify motor benefits but also increase the risk of dyskinesia.
Special Populations
Tailoring therapy is essential for certain groups:
- Renal impairment: Reduce dose proportionally to creatinine clearance; monitor for neuropsychiatric symptoms.
- Elderly: Start at the lowest effective dose (50 mg once daily) and assess tolerance before titrating.
- Pediatric: Use weight‑based dosing for antiviral prophylaxis; avoid in children under 1 year due to limited safety data.
Frequently Asked Questions
What is the typical onset time for amantadine’s effect in Parkinson’s disease?
Patients often notice improvement in rigidity and bradykinesia within 1-2 weeks of starting therapy, although full benefit may take up to a month.
Can amantadine be used for COVID‑19?
Current evidence does not support amantadine as an effective treatment for SARS‑CoV‑2 infection. Its antiviral activity is specific to the influenza A M2 channel.
How should the dose be adjusted in a patient with a creatinine clearance of 30 mL/min?
A common approach is to halve the usual daily dose (e.g., 100 mg once daily) and monitor for signs of accumulation.
Is it safe to combine amantadine with levodopa?
Yes, the combination is frequently prescribed. Amantadine can reduce levodopa‑induced dyskinesia, but clinicians should watch for additive CNS side effects.
What are the signs of amantadine toxicity?
Severe toxicity may present as agitation, hallucinations, seizures, cardiac arrhythmias, or marked renal dysfunction. Prompt dose reduction or discontinuation is required.
